Gene therapy: Discussing the world’s most expensive treatments at Health Horizons Future Healthcare Forum
#Gene_therapy: Discussing the world’s most expensive treatments at Health Horizons Future Healthcare Forum
International #Conference on #Genetic_Disorders and #Gene_Therapy
#August 26-27, 2019 #Bangkok, #Thailand
Theme: Current research and futuristic challenges in Genetics
Email us : geneticdisorders@asiapacificmeets.com
Contact us : https://genetics.geneticconferences.com/
Register Now...!!!!!!
A new wave of treatments is coming in which a patient's genetic code is altered. But these one-off therapies can have huge price tags, prompting discussion over their affordability and accessibility. Editor Paul Brackley examines the issue, which he will discuss in a fireside chat at the Health Horizons Future Healthcare Forum with Steve Altschuler, chairman of Spark Therapeutics, on Wednesday June 26.

The most expensive drug ever was approved last month by the US Food and Drug Administration (FDA).
Priced at $2.125million, Zolgensma is a gene therapy from the company Novartis for the treatment of spinal muscular atrophy in children under the age of two.
The company’s CEO, Vas Narasimhan, said: “The approval of Zolgensma is a testament to the transformational impact gene therapies can have in reimagining the treatment of life-threatening genetic diseases like spinal muscular atrophy.”
Caused by a defective or missing SMN1 gene, infants with this rare, devastating condition lack the motor neurons needed for muscle functions such as breathing, swallowing, speaking and walking.
In its most severe form, the disease leads to paralysis and ultimately permanent ventilation or death by the age of two in 90 per cent of cases.
With one dose of Zolgensma, a functional copy of the human gene helps to stop the disease progressing by enabling sustained expression of the SMN protein. In trials, it extended children’s lives and many were able to to sit, roll, crawl and play. Some were able to walk.
Jerry Mendell, principal investigator at the Center for Gene Therapy in Columbus, Ohio, said: “This level of efficacy, delivered as a single, one-time therapy, is truly remarkable and provides a level of unprecedented hope for families battling SMA type 1.”
It is a striking example of the exciting potential of gene therapy - technology that scientists have been talking about since the 1970s, but which has only recently become a reality, prompting discussion around the affordability and accessibility of such expensive treatments.
Many diseases have an underlying genetic cause. The aim of gene therapy is to correct this by altering a person’s genetic code - by replacing a faulty or missing gene, for example.
Although only a few gene therapies are so far approved, there are more than 1,000 different types in clinical trials and they use a variety of strategies - adding genes, correcting them, ‘silencing’ them, using genes to reprogram cells, or deploying ‘suicide genes’ to cause the death of certain harmful cells, for example.
A typical approach is for a therapeutic gene to be delivered into the body via a carrier, known as a ‘vector’. This can be a genetically-altered virus that infects the target cells and then releases its genetic payload.
The gene can be used to carry, for example, the instructions needed to create a functional protein, restoring the cell to normal working order.
“Gene therapy opens up a whole new world. A lot of new drugs in the area are going to be approved,” the chairman of Spark Therapeutics, Steve Altschuler, tells the Cambridge Independent.

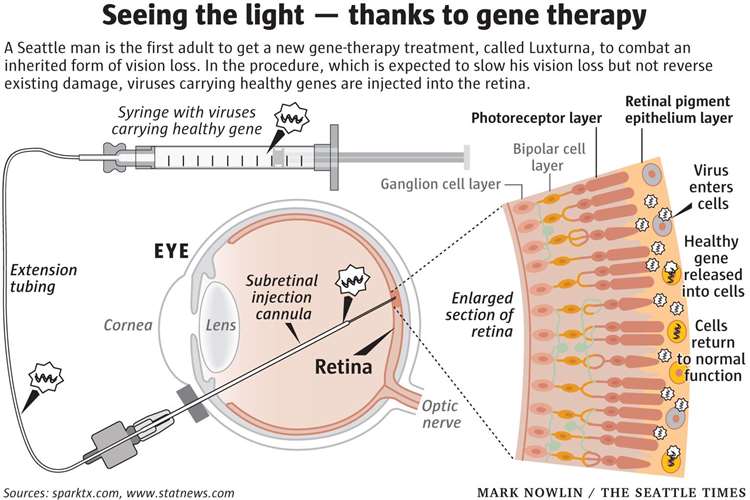
On December 19, 2017, the company’s drug Luxturna became the first directly administered gene therapy approved in the United States that fights a disease caused by a mutation in a specific gene.
It treats a form of retinal dystrophy that leads to vision loss and may cause complete blindness in certain patients.
It does so by delivering a normal copy of the RPE65 gene - mutated in patients with this disease - directly to retinal cells.
It was released with a price tag of $850,000, or $425,000 per eye. But then, what price would you place on vision?
“The cost is high because of the development timeframe,” says Steve, who will discuss the issue of making such innovative drugs accessible in a fireside chat at the Health Horizons Future Healthcare Forum in Cambridge on Wednesday (June 26).
“The drug that we developed was 25 years in development from the earliest stages of this field. The expense associated with getting the drug approved was literally hundreds of millions of dollars and this particular drug treats an ultra-rare disease.
“So if you take the development cost and divide it by the number of patients it becomes quite expensive.
“This first drug was really to prove you could use gene therapy to treat disease. The market for it is so small that you can’t make enough money to support the company long-term.
“We have to advance to other therapies where the market is bigger and the commercial potential is bigger.
“But drugs in these categories tend to treat diseases that are ultra rare and with high development costs.”
While the upfront costs of gene therapies are substantial, their value needs to be put in context.
Typically one-off doses that aim to cure, or significantly alleviate, genetic diseases, their price needs to compared to the costs of ongoing treatment with more traditional drugs.
And when the life-changing impact on a patient is considered - along with the social and economic benefit of curing them - gene therapies start to look very attractive.
Spark Therapeutics, recently acquired by Roche for $4.3billion, is developing treatments for haemophilia A and B.
“Haemophilia in most patients costs $500,000 a year,” says Steve. “Even if you develop a gene therapy that costs $2million, you’ve saved the patient, insurance company, or government - whoever the payer is - with a one-time dose, compared to taking a drug for 30 years.
“Ultimately, the cost to society and to the patient with these one-time curative therapies become much less if you look at it over the course of a lifetime.
“Then there is a contribution to society. Think about curing blindness in a child - what that means for their life, and then in their ability to be productive.”
With Luxturna, Spark Therapeutics has taken a novel approach to payment.
“What we decided to do with this first indication is go for an outcome-based payment. You give the patient gene therapy. If they have restoration of sight six months after the injection, we get half the payment. We get the rest of the payment if the patient still has their sght after two or three years depending on the contract,” says Steve.
“The drug is rare enough and the patient population is small enough that the insurance companies can manage it.
“Some cancer immunotherapies have also gone for this kind of payment mechanism. It helps to justify and show the value of these very expensive one-time therapies.”
Luxturna, licensed to Novartis outside the US, has won European Commission approval. In the UK, the National Institute for Health and Care Excellence (NICE) is examining whether to recommend it for use in the NHS based on its cost effectiveness.
In the US, the situation is more complicated, as people can change their health insurance regularly.
Will an insurer want to offer cover for a highly expensive one-off treatment?
“That’s always been a problem in our system compared to the UK’s, where there is a single payer in the NHS,” notes Steve.
“In the States, there is the notion of a reinsurance pool, whereby insurers can buy additional insurance for these very expensive cases.
“If people change jobs and their insurance product every five years, it becomes very difficult to amortise the cost of these.
“Insurance companies are trying to come up with these reinsurance products that multiple insurance companies can invest in and smooth out this cost over time. They are effectively shipping risk out to a super insurer that sells insurance for these very high cost therapies.”


